Think
Try to imagine an image of a mixed orchard with different kinds of trees. How would you attempt to differentiate and describe each tree or group of trees?

Notebook
Based on the image you've created in your head, answer the following questions then compare to another student's suggested answers. Don't worry if your answers don't match – the goal here is to think about how you would go about categorizing the trees in your imaginary orchard.
Reflect and answer the following questions in your notebook.
1. What are some terms that would be the same for each tree?
- fruit tree
- tree planted by humans
2. What are some terms that would be the same for a group of trees (but not all trees)?
- apple tree
- pear tree
3. What are some terms that would be unique for an individual tree?
- the single McIntosh apple tree among the apple trees
- the only cherry tree in the orchard
An example of a description of an individual tree might be:
"The 100-year old walnut tree planted by my great grandfather, struck and almost burned down by lightning last year, which always loses its leaves last in the fall and blooms earliest at springtime, etc."
In this activity, you will learn how chemists draw and describe an organic compound by the name that they use. Just as you described the features of the trees, chemists describe the features of a compound using terms that are common to a class of compounds as well as unique to the individual compound itself.
The representation of organic compounds
There are a few different ways in which chemists represent organic structures. Three of them are:
- complete structural formulas (also referred to as complete formulas or structural formulas)
- condensed structural formulas (also referred to as condensed formulas)
- line diagrams (also referred to as skeletal formulas)
You need to be familiar with each of these ways of representing organic compounds, in order to be able to recognize and name them.
Notebook
Make sure to include summaries of each type described in your notebook as you press the following phrases.
Try it!
Now that you have learned about complete formulas, condensed formulas and line diagrams, complete the following chart in your notebook. When you are ready, check your answers.
Discover more
We experience hydrocarbons (organic compounds containing hydrogen and carbon) everywhere in our life, often without realizing it.
Using the internet or any other resources available to you, find the complete structural formula for:
- Methane – a greenhouse gas that is found in the flatulence of cows (and humans too)
- Propane – the gas used in portable barbecues
- Butane – the gas used in portable lighters
- Hexane – the gas used to extract oils from seeds and vegetables
Notebook
In your notebook, draw or copy and paste a picture of each of the compounds mentioned in Discover more, write their name beside the picture and identify similarities in the names and structures.
Alkanes
The examples – namely methane, propane, butane and hexane – are organic compounds that are classified as alkanes. All alkanes share similar structural properties:
- The carbon atoms have single bonds to other carbon atoms.
- They have a general formula of .
Just as we could describe the trees in the “Minds On” section at the beginning of this activity, chemists use the name of compounds to describe them.
For example:
| Propane | Butane |
|---|---|
|
Prop-: means a three-carbon chain -ane: means all single bonds |
But-: means a four-carbon chain -ane: means all single bonds |
So you now know that the ending “-ane” means that it is a compound composed of all single bonds, but what are the prefixes that are added to indicate the number of carbons?
Prefixes indicating the number of carbons in an alkane
| Prefix | Number of carbons | Prefix | Number of carbons |
|---|---|---|---|
| Meth- | 1 | Hex- | 6 |
| Eth- | 2 | Hept- | 7 |
| Prop- | 3 | Oct- | 8 |
| But- | 4 | Non- | 9 |
| Pent- | 5 | Dec- | 10 |
So, heptane is a 7-carbon chain made of all single bonds and looks like this:

Notebook
In your notebook, draw the structural forumulas of all the alkane compounds in the table. You may also use a digital drawing tool. When you are ready, press Show Answers to verify your drawings.
You now know that the name given to an organic compound describes the structure of the compound, so now, let’s explore more complex structures.
Substituted alkanes
Consider the following structure:

In order to name this structure, we need to describe everything about it including:
- the number of carbons that are in the chain
- the fact that there is an additional carbon that branches off of the chain
Press the following tabs to examine these pieces step by step.
Try it!
Complete the following table in your notebook. When you are ready, check your answers for understanding.
Alkanes with more than one substituent group
What about compounds where there is more than one substituent group?
For example this compound:

We will use the same questions and steps as before:
- What is the length of the longest chain?
- What are the substituent groups?
- Where are the substituent groups located?
Press the following tabs for step by step process.
When there are multiple substituents of the same type (e.g. 2 methyl groups), you identify the carbon number they each branch from, with the numbers separated by commas, and then you use a prefix of di-, tri-, tetra-, etc.
For example:
Example 1

2,3-dimethylpentane
Example 2

3,3-dimethylpentane
In example 2, note that even though the methyl groups are on the same carbon, they are numbered twice (e.g. 3,3). This is to make sure it is clear that both methyl groups are on the same carbon.
The di-, tri-, etc. prefixes don’t affect the alphabetical order of listing the substituent groups.
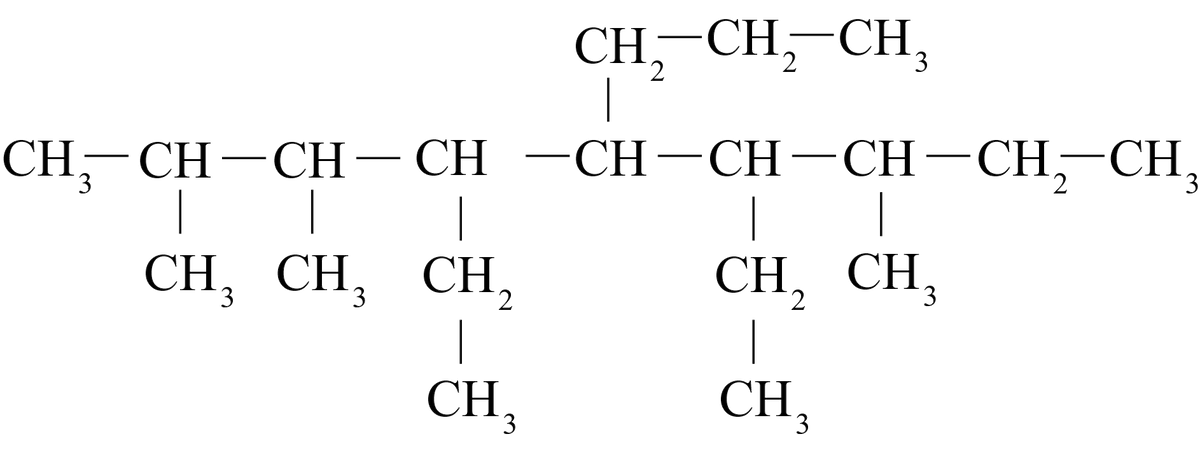
Consider the following compound:

This compound is correctly named 4-ethyl-3,5-dimethylheptane and not 3,5-dimethyl-4-ethylheptane.
Try it!
Name the following compounds. Carefully follow the steps as shown in the previous examples. When you are ready, check your answers.
Note that it is very important to use:
- the proper numbering for the main carbon chain
- dashes between a number and a name
- commas between two or more numbers
Join the discussion
In the discussion forum, draw the structural formula for 3-ethyl-5-methylheptane. You may use any drawing tool of your choice. Do you have any advice on tips on using your drawing app? Please share with the class.
Notebook
In your notebook, draw the structural diagram for methylbutane. Alternatively, you may choose to use a digital drawing tool.
Why do you think a number isn’t required in front of the methyl substituent in methylbutane?

Specifying the position of the methyl group in methylbutane isn’t necessary (although it can be done), because there is only one possible place where a methyl side chain can be added: on the second carbon atom. Even if the methyl group is moved one to the right in the diagram, beside the methyl group would still be at carbon 2, as the main carbon chain would have to be numbered from the right to the left. Moving the methyl group to either end of the main carbon chain would now make the longest carbon chain to be five long, and the compound would now be pentane.
Structural isomers
Using a line diagram, represent in as many unique ways as you can in your notebook. When you are ready, check your drawings.

Figure a)

Figure b)

Figure c)

Figure d)

Figure e)
The compounds represented in the figures above are known as structural isomers of each other. Structural isomers are compounds that have the same molecular formula (in this case ) but different physical arrangements of atoms.
Try to name all the represented isomers represented in the previous figures. Check your answers when you are ready.
Figure a): 3-methylpentane
Figure b): hexane
Figure c): 2-methylpentane
Figure d): 2,3-dimethylbutane
Figure e): 2,2-dimethylbutane
Think
Why isn’t the compound shown in the following Figure f) also one of the possible structural isomers of ? To help you answer this question, name the compound. Write the name and your reasoning in the space provided. Then, verify your answer.

Figure f)
The molecule represented in Figure e), 2-methylpentane, is the exact same compound as that represented in Figure c), which is also called 2-methylpentane. They are just mirror images of each other. Therefore, since it is the same compound (as evidenced by the shared name) it is only listed once as a possible structural isomer of .
Other substituent groups
Organic compounds often contain substituent groups that aren't hydrocarbons. To name these compounds we use prefixes to identify the different substituent groups. Complete the following table of prefixes using the internet or other resources available to you.
| Substituent | Prefix |
|---|---|
| Cl | chloro- |
| Br |
bromo- |
| F |
fluoro- |
| I |
iodo- |
| NO2 (as a substituent) |
nitro- |
Notebook
In your notebook, draw the compound and explain why you named it as you did. You can also use a digital drawing tool as well. When you are ready, check your answer.
Using your knowledge of the rules for naming alkanes, what would you name the following compound:

Name: 2-chloropentane.
Explanation: The parent chain of this compound is 5 carbons, therefore the root name is pentane. There is a chlorine atom found on the second carbon atom in the chain, therefore the compound is given the prefix 2-chloro.
Notebook
Practise your ability to name the following compounds. For each compound, select the corresponding name description, from the drop-down menu. Then press Submit to check your understanding.
Notebook
Finally, try to name the compounds in your notebook. When you are ready, verify your answers.
Join the discussion
Refer to Learning Activity 1.1, where you identified an organic compound that was used in the industry. Research the structure of the compound and identify any substituent groups that have been introduced in this unit. Post your structural and list all of the substituent groups that are part of the organic compound.
Reflection
Review the learning goals and success criteria for this learning activity. Revisit any areas you need to strengthen your understanding. Reflect on the following success criteria and make note of your responses in your notebook.
Success criteria
Am I able to...?
- represent an organic compound in a variety of ways
- name straight alkanes
- name alkanes that have substituents attached (e.g. a methyl-group or a chloro-group)
- recognize a structural isomer of a compound
- create, name and draw structural isomers of a chemical formula