What is an atom?
Thoughtbook task: Mystery box
Have you ever received a wrapped present and tried to guess what was inside? How would you go about determining what was in it? What are some strategies and skills you might use? Take some time now to record your answers in your Thoughtbook.

How would you feel if you could shake the box and measure it, but never got to open it? What if you had to do your best to figure out what was in the box without unwrapping it? That was the task undertaken by scientists of the 1800s and early 1900s who studied the atom.

Scientists studying the atom tried to determine the structure and behaviour of something too small for them to actually observe. Nobody knew the right answer to their questions, so they could only do their best to figure out what was happening on the atomic scale of matter. The collective achievements that uncovered the atom’s structure are one of science’s major accomplishments.

Ernest Rutherford with the experimental set-up used to determine an atom’s structure.
Thoughtbook
You have likely learned some things about atoms in your previous science courses, and are starting this course with your own ideas of what atoms are.
Draw a sketch of an atom in your Thoughtbook based on what you currently understand about the atom. Include labels on your sketch if you think it is appropriate.
You will revisit this sketch later in this learning activity to make modifications and revisions based on what you have learned.
Let’s now begin our exploration of atomic theory.
This learning activity is separated into four parts:
Action Part 1: Exploring the particle theory of matter
Action Part 2: Examining the states of matter
Action Part 3: Observing the structure of the atom
Action Part 1: Exploring the particle theory of matter

Chemistry is the study of matter and its properties. In this unit you will deepen your understanding of the structure of matter and how that structure determines chemical behaviour. Understanding chemical behaviour will help you learn how to use chemicals in a way that benefits society and the environment, as well as how to use them safely to avoid causing potential harm.
Important
The particle theory of matter is the accepted scientific model of the structure of matter. According to particle theory:
- All matter is made up of tiny particles.
- All particles that make up a pure substance are the same.
- Different substances are made of different types of particles.
- Particles are always in motion, and move faster when they have more energy.
- All particles are attracted to other particles. (Forces are stronger when particles are closer together.)
Notebook
Now take some time to record in your own words the five “postulates” of the particle theory. Consider drawing a sketch for each postulate.
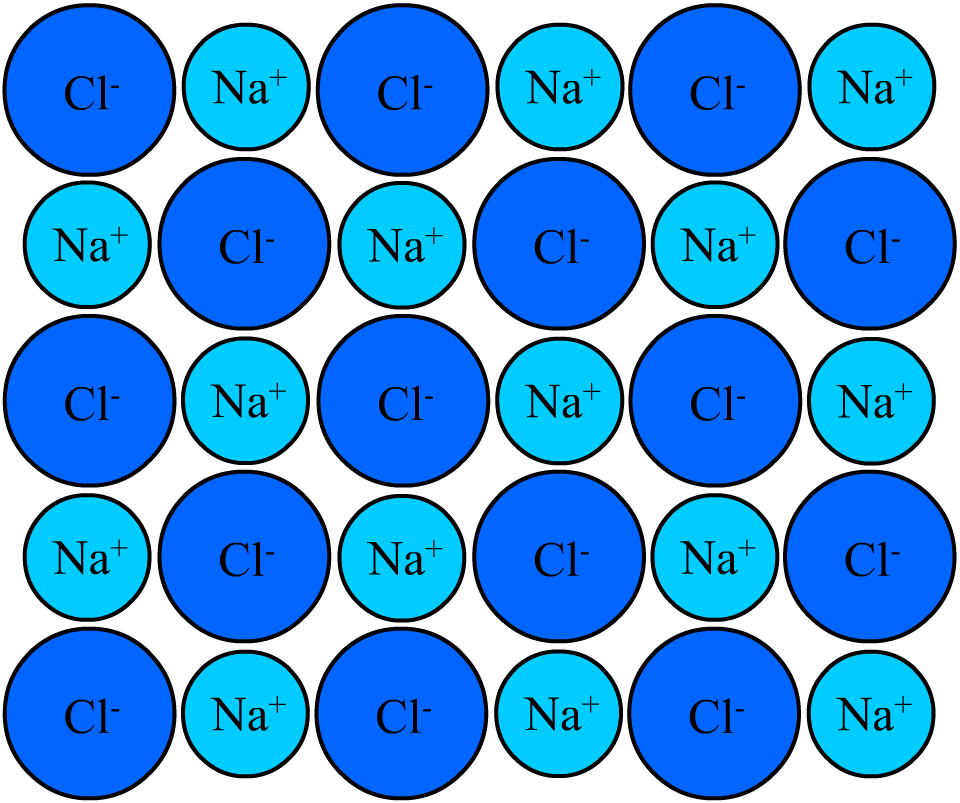
The particles that make up matter are so small that it is difficult for anyone to imagine how tiny they are. If you shake a single crystal of salt into your hand and examine it, you will find a small transparent cube. The number of atoms in the cube would be more than a billion, billion, billion atoms. If you wrote a one with twenty zeros (100,000,000,000,000,000,000), you would have a number close to the number of atoms in the single grain of salt!

Types of particles
The three types of particles that make up matter are atoms, molecules, and ions. Atoms are the smallest particles of matter and they can bond to one another to form molecules. Atoms and molecules that have an electric charge are called ions.
Notebook
Read the summary about each type of particle. In your notebook, write a brief summary about each type in your own words. Include examples that will help you deepen your learning toward each type of particle.
Try it!
Answer the following questions in your notebook in order to check your understanding of particle theory and the three types of particles. Once you are confident with your answers, compare them with the suggested ones.
Action Part 2: Examining the states of matter
The particle theory of matter can help us understand the states of matter (solids, liquids, and gases) that we observe around us. Recall the fourth and fifth postulates of the particle theory from earlier in the learning activity:
- Particles are always in motion, and move faster when they have more energy.
- All particles are attracted to other particles. (Forces are stronger when particles are closer together.)
Notebook
Take a moment to think about how the particle theory of matter can help describe states of matter.
Based on what you understand about the states of matter, how can particle theory help explain the existence of solids, liquids, and gases? Can particle theory explain phase changes (melting, freezing, evaporation, and so on)? Jot down any ideas or questions you have on this topic in your notebook.
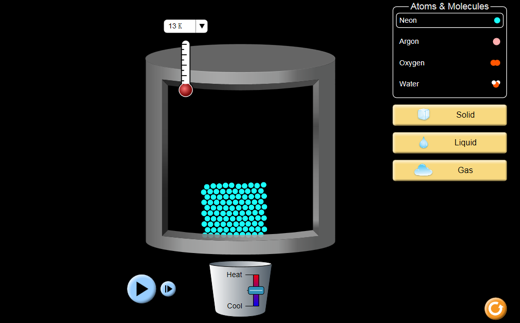
States of matter simulator
The following simulation allows you to explore the effects of heating and cooling on particle motion.
Instructions:
Once you have started the simulation, choose “States.” The temperature scale can be changed to Celsius using the drop-down arrow on the thermometer. Use the menu on the right to choose a chemical substance and its state. You can use the temperature control to change the substance’s state. Observe the behaviour of the particles as the temperature changes and record your observations in your notebook. While you are using the simulation consider what you wrote in your notebook. Do your observations in the simulation support your ideas? Can you add to your ideas or answer any questions you wrote down?
Notebook
States of matter observation table
| Atom or molecule | State | Temperature (K) | Motion of particles | Organization of particles | Proximity of particles |
|---|---|---|---|---|---|
Press here (Opens in new window) for an accessible version of States of matter simulation.
Press the image below to open the simulator.
States of matter observations
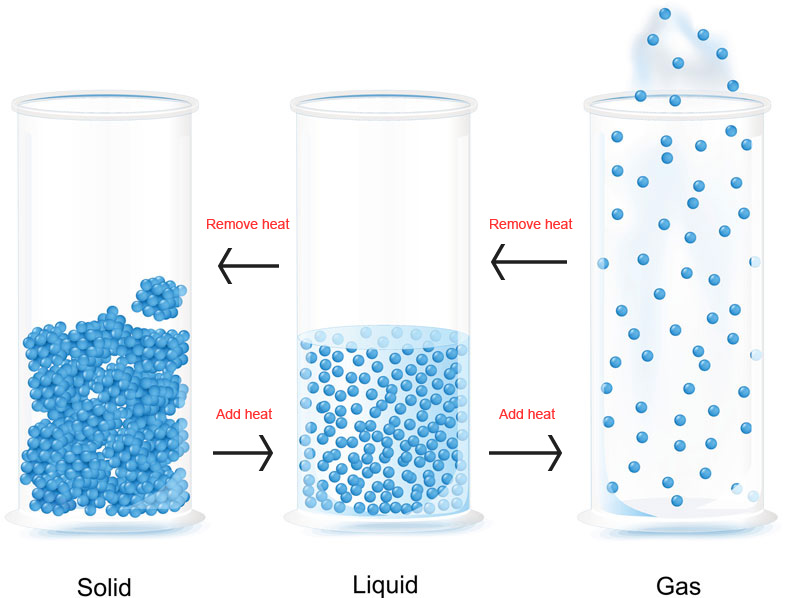
From your experience with the simulator, you may have observed how the relative strength of attraction between particles can be demonstrated by how difficult it is to melt or boil a substance. Temperature changes make particles move faster or slower and allow them to move apart or together. If they move apart or together, the state will change.
If particles are moving more slowly (colder), their attractive forces have a stronger effect; if they are moving faster (hotter), their attractive forces have a weaker effect. If the attractive force between particles is naturally large, the substance is likely to be a solid and heat will be needed to melt it. If the force is weak, the substance will not be solid and the particles will have to be cooled down (move slowly) to make the attraction take effect.
The effect of heat on changes of state is shown in this diagram:
As you continue this learning activity, you will take steps to deepen your learning of the molecules that make up matter.
Action Part 3: Observing the structure of the atom
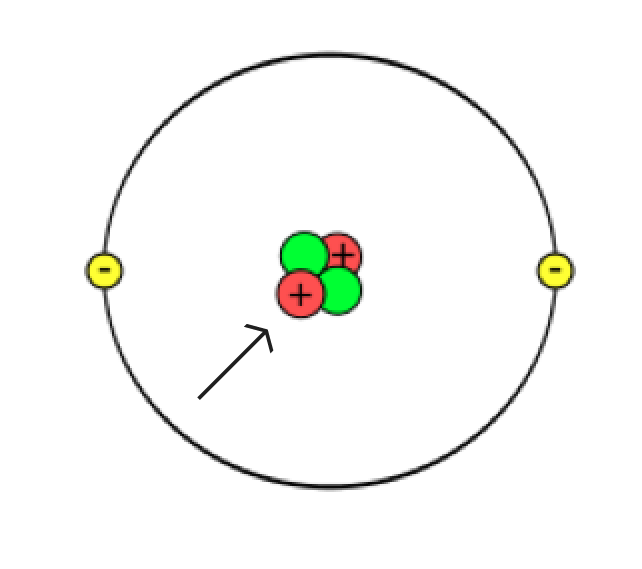
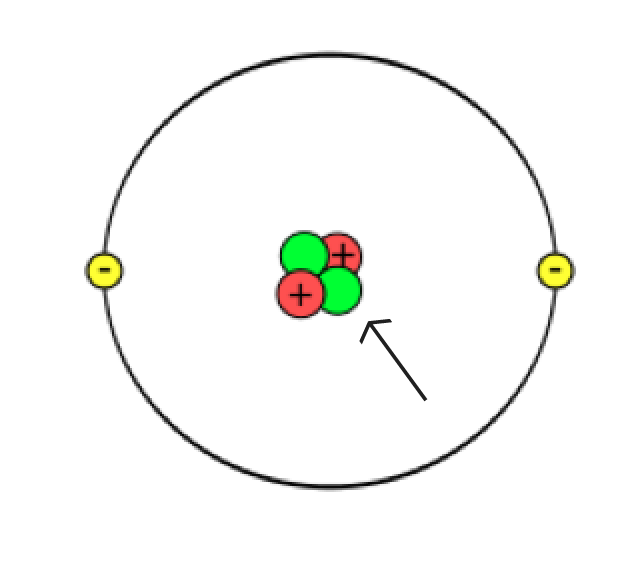
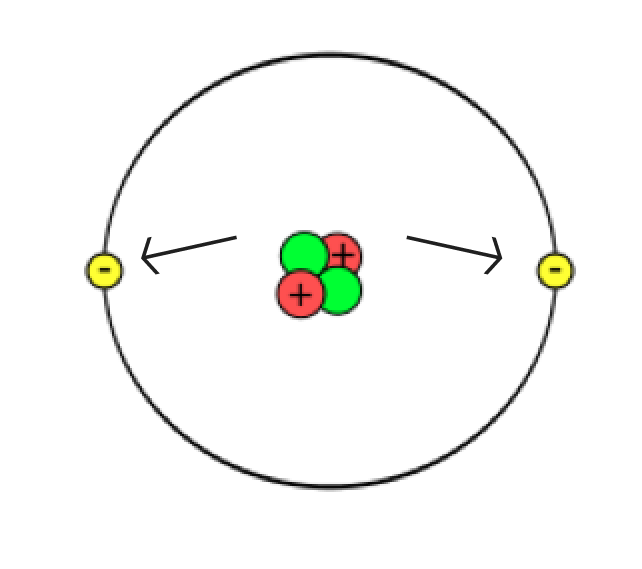
As you have already read, atoms are very small and very numerous. Many scientists have contributed to our understanding of atomic structure. Based on the results of their work, we can explain the atom as follows:
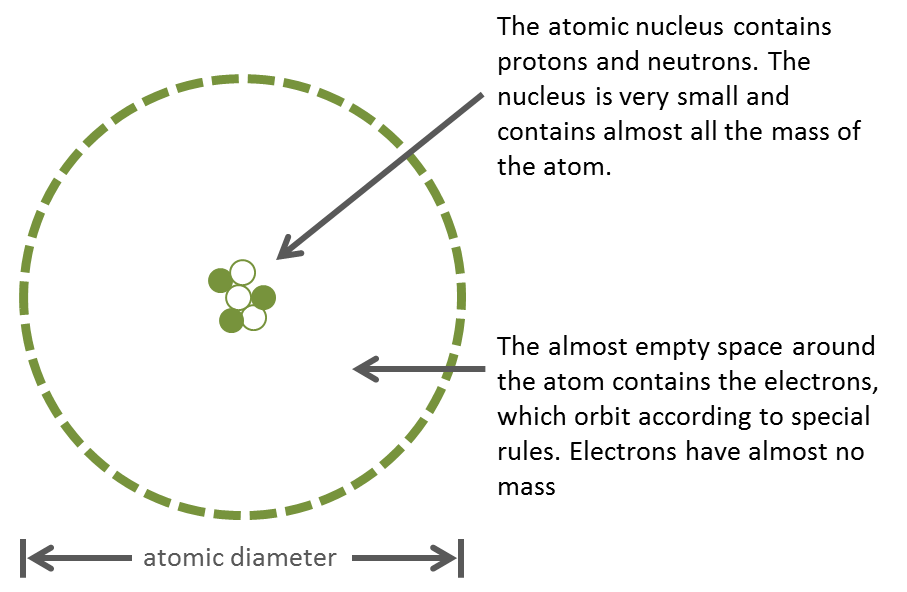
The nucleus is very small, as small as 1/100,000 of the size of an atom’s diameter. To put these dimensions to scale, if the nucleus were the size of a green pea, and you placed the pea on the pitcher’s mound at the baseball stadium in Toronto, the atom would be the size of the entire baseball stadium! Despite its tiny size, the nucleus has more than 99% of the entire atom’s mass.

Notebook
Copy the following table into your notebook.
Complete the following chart for the three subatomic particles. When you have finished, compare your answers by pressing 'Suggested Answers'.
| Particle name | Particle symbol | Particle change | Particle masses (u) | Location in the atom |
|---|---|---|---|---|
| proton | p+ |
+1 |
1.0 |
nucleus |
| electron | e- |
-1 |
0.00054 or 1/2000 |
energy levels |
| neutron | n0 |
neutral |
1.0 |
nucleus |
Thoughtbook
Structure of the atom
Revisit the sketch of the atom you made earlier in this learning activity.
Which parts of your sketch are accurate? Which parts need changing? Are there additional things you can add to your sketch?
Make any changes to your sketch that you feel are necessary and then jot down a brief list of the things you changed on your original sketch.
Symbols on the periodic table of elements
The periodic table will be useful as a reference for this learning activity and throughout the course, and especially when completing support questions and assessments. You may wish to save and/or print it for easy reference in the future.
Download the “Periodic Table of the Elements (Opens in new window)” and become familiar with the information provided within it.
Additionally, you may choose to explore the following interactive periodic table by observing the different characteristics and organization of the elements within.
Press here (Opens in new window) for an accessible version of the Periodic table.
Characteristics of the nucleus
Scientists have found it helpful to define two nuclear characteristics: the atomic number and the mass number.
The atomic number (Z) is the number of protons in the nucleus and it is the element identifier. The mass number (A) represents the total particles found in the nucleus. It is found by adding the number of protons to the number of neutrons. (Note: The atomic mass number is the rounded value of the atomic mass of an atom.)
If you know the number of protons and neutrons – for example, silver has 47 protons and 60 neutrons – then the mass number for the silver nucleus will be 47 + 60 = 107. Conversely, if you know the mass number, you can find the number of neutrons by subtracting the atomic number from the mass number.
Important
Atomic Number = # of protons.
Mass Number = # of protons + # of neutrons.
Let’s give it a try!
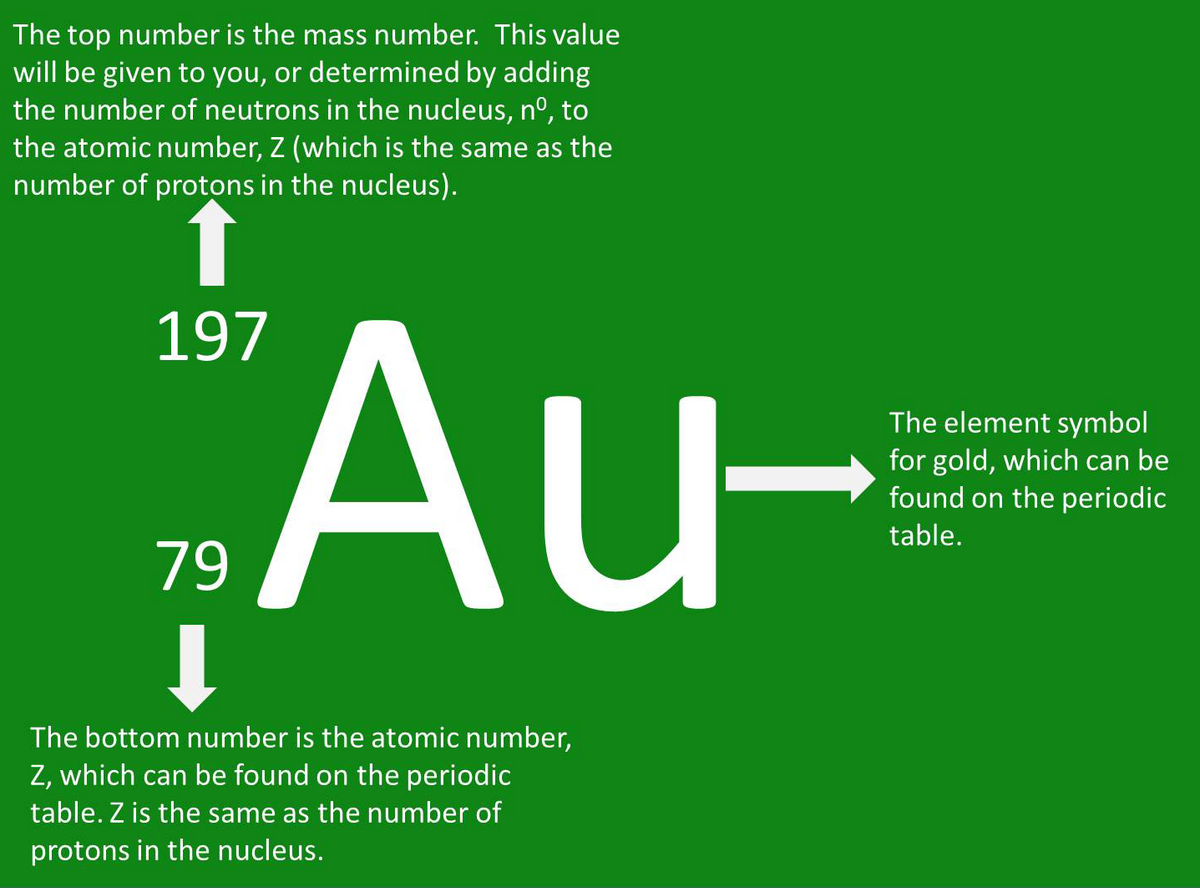
You will often find the values for the atomic number and the mass number placed as subscripts and superscripts beside the element’s symbol. For example, the nuclide symbol for gold (Au) is shown in the following image.
Try it!
Create a table with the following headings in your notebook, including the example provided. Leave room for four additional rows of information:
| Element name and symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Nuclide symbol |
|---|---|---|---|---|---|---|
| phosphorus (P) | 15 | 31 | 15 | 15 | 16 |
Using your “Periodic Table of the Elements (Opens in new window)” and the information provided here, complete the table for these four elements:
- copper (Cu, atomic mass = 64)
- barium (Ba, atomic mass = 137)
- iodine (I, atomic mass = 127)
- potassium (K, atomic mass = 39)
Once you have completed the table, compare your answers with these suggested answers:
| Element name and symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Nuclide symbol |
|---|---|---|---|---|---|---|
| copper (Cu) | 29 | 64 | 29 | 29 | 35 |
| Element name and symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Nuclide symbol |
|---|---|---|---|---|---|---|
| Barium (Ba) | 56 | 137 | 56 | 56 | 81 |
| Element name and symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Nuclide symbol |
|---|---|---|---|---|---|---|
| iodine (I) | 53 | 127 | 53 | 53 | 74 |
| Element name and symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Nuclide symbol |
|---|---|---|---|---|---|---|
| potassium (K) | 19 | 39 | 19 | 19 | 20 |
As we continue this learning activity, we will learn how different elements can actually have numerous mass numbers depending on the makeup of the nucleus.
Isotopes
Until the early 1900s, it was believed that all atoms of a single element were identical. In fact, this is not the case! Even though all potassium atoms must have 19 protons and electrons, they do not have to have the same number of neutrons.
Atoms of the same element that contain different numbers of neutrons are called isotopes.
Isotopes of an element have all the same chemical characteristics, and differ in mass only. Isotopes are very important to medicine, nuclear energy, and industry.
Explore this!
Explore the video “Isotopes,” which summarizes the information about isotopes. While you are exploring the video, feel free to jot down any notes in your notebook.
Radioisotopes
Radioisotopes are nuclei that have an unstable nucleus. As a result of that instability they break down, releasing particles of energy from the nucleus, called radiation. Some radioisotopes (radioactive isotopes) occur in nature and some are produced in nuclear reactors.
Radioisotopes like cobalt-60 and sodium-24 are used in medicine and industry because of the radiation they give off.
Notebook
Isotope and radioisotope
In your notebook, ensure that you have written a definition for isotope and radioisotope in your own words.
Isotopes: researching examples and uses
Notebook
In your notebook, create a table like the one below, including the example provided. Note how, for isotopes, the mass number (A) of the isotope is indicated within the isotope name.
Using print or electronic resources, research any four isotopes used in medicine or industry and explain their use.
Having trouble getting started? Try researching one or more of the following isotopes: hydrogen-3, carbon-14, cobalt-60, fluorine-18, sodium-24, uranium-238.
| Isotope name | Nuclide symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Use |
|---|---|---|---|---|---|---|---|
| hydrogen-1 | 1 | 1 | 1 | 1 | 0 | Normal hydrogen gas used as a fuel and as an agent for hydrogenation of oils into margarine |
| Isotope name | Nuclide symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Use |
|---|---|---|---|---|---|---|---|
| hydrogen-1 | 1 | 1 | 1 | 1 | 0 | Normal hydrogen gas used as a fuel and as an agent for hydrogenation of oils into margarine | |
| hydrogen-2 (deuterium) | 1 | 2 | 1 | 1 | 1 | Heavy hydrogen, as part of water, used as a moderator in nuclear reactors. | |
| carbon-12 | 6 | 12 | 6 | 6 | 6 | Normal carbon used for charcoal and in all organic molecules | |
| carbon-14 | 6 | 14 | 6 | 6 | 8 | Radioactive carbon formed naturally in the atmosphere and used for carbon dating |
| Isotope name | Nuclide symbol | Atomic number (Z) | Mass number (A) | # of protons | # of electrons | # of neutrons | Use |
|---|---|---|---|---|---|---|---|
| cobalt-60 | 27 | 60 | 27 | 27 | 33 | Radioactive cobalt manufactured in a nuclear reactor and used to kill cancer cells | |
| sodium-24 | 11 | 24 | 11 | 11 | 13 | Radiosodium also made in nuclear reactors and used to follow circulation of blood | |
| uranium-238 | 92 | 238 | 92 | 92 | 146 | Uranium isotope present in largest amount | |
| uranium-235 | 92 | 235 | 92 | 92 | 143 | Uranium isotope present in much smaller amount and used for reactors and nuclear weapons |
Average atomic mass
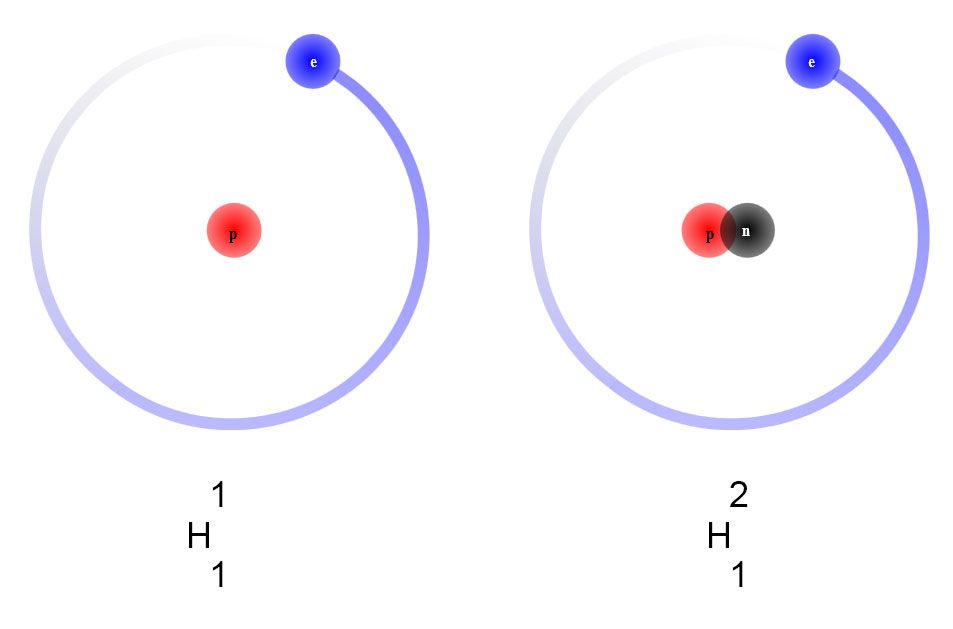
Elements like hydrogen, carbon, and uranium exist in nature as a mixture of isotopes. These isotopes are present in different percentages in the natural element. For example, hydrogen-1 (normal hydrogen) has a natural percentage of 99.98%, the rest being hydrogen-2, heavy hydrogen, or deuterium. In the case of uranium, isotope uranium-238 has an abundance of 99.28% and isotope uranium-235 has an abundance of 0.71%. For chlorine, isotope chlorine-35 has an abundance of 75.77%, and the rest is isotope chlorine-37.
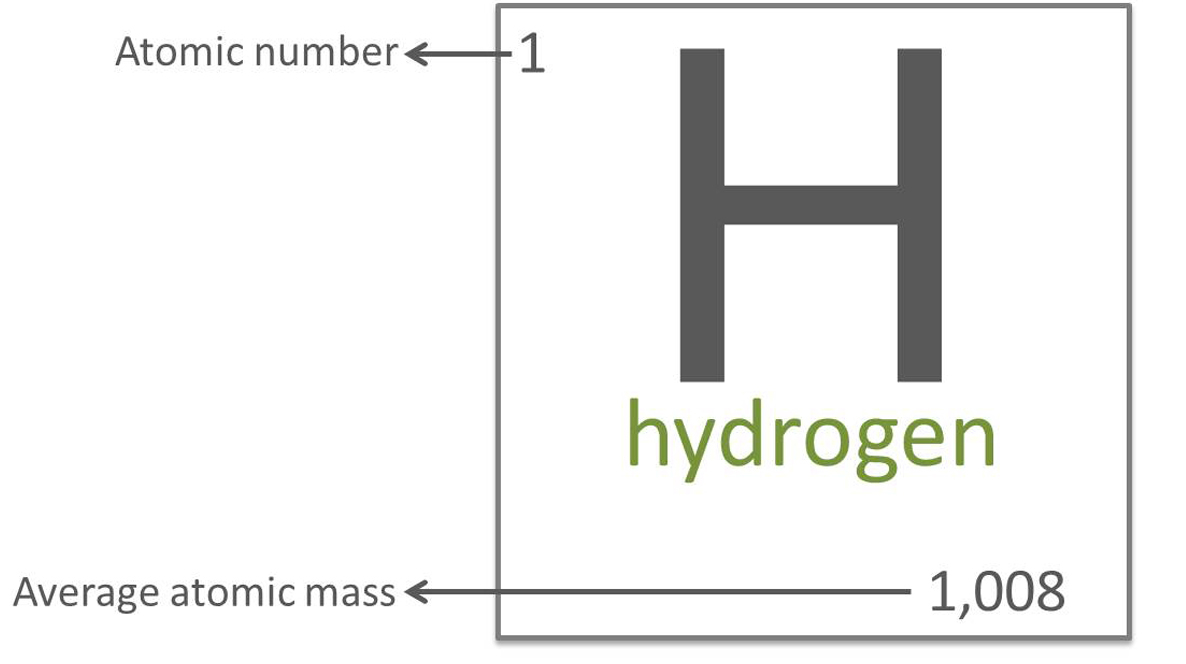
Since elements consist of a mixture of natural isotopes, samples of an element will have an average mass based on the masses of the isotopes and their percentage presence. The periodic table lists this average mass. The average mass of hydrogen is 1.008 u, since most of the hydrogen has a mass of 1.0 and a small amount has a mass of 2.0.
In the case of carbon, the average mass is 12.011 u. Since the majority of carbon found in nature is carbon-12, the average atomic mass value is close to 12. Chlorine has an average mass of 35.453 u because, while most of the chlorine is chlorine-35, about 25% of chlorine is chlorine-37.
Explore this!
Explore the video The Nucleus: Crash Course Chemistry #1 for a summary of the information on relative atomic mass. As you are exploring the video, take the opportunity to use your notebook to jot down any notes.
Notebook
For the following questions, write the answers in your notebook. Once you have finished, press 'Suggested Answer' to compare your answers with those shown.
Boron has two isotopes, boron-10 and boron-11. If the average atomic mass of elemental boron is 10.815 u, which isotope is in the largest abundance?
Isotope boron-11. Since the average atomic mass is 10.815, this is closer to 11 than to 10, so there must be a larger contribution from isotope boron-11.
The average atomic mass of magnesium is 24.305 u. The element has three natural isotopes: magnesium-24, magnesium-25, and magnesium-26. Which of the isotopes is likeliest to be in the largest amount?
Isotope magnesium-24. Since the average mass is 24.305, this is closest to isotope magnesium-24 and therefore that isotope is in greatest abundance.
Hydrogen has a third isotope that is radioactive and has almost no natural abundance. Do a search of the isotopes of hydrogen on the Internet or in a library to find out the name of this isotope and how many protons, electrons, and neutrons it has.
The third isotope of hydrogen is called tritium. Because it is hydrogen, it has one proton and one electron. With a mass number of 3, the isotope has two neutrons.
Radioiodine, iodine-131, has medicinal use in diagnosing a particular disease. Do a search for iodine-131 on the Internet or in a library to find out what it is used for and how it can be dangerous.
Iodine-131 is used to diagnose diseases of the thyroid gland. This isotope can be dangerous. Since iodine accumulates in the thyroid gland, too much iodine-131 can cause thyroid cancer. This was a problem in the population living near the nuclear disaster in Chernobyl and is a hazard for those living in areas where nuclear weapons are tested.
Career Connection
A career in relation to the study of chemistry is a Nuclear Chemist. The Federal Government of Canada has provided the following description for this unique career:
Chemists conduct research and analysis in support of industrial operations, product and process development, quality control, environmental control, medical diagnosis and treatment, biotechnology, nanotechnology and other applications. They also conduct theoretical, experimental and applied research into basic chemical and biochemical processes to create or synthesize new products and processes. They are employed in research, development and quality control laboratories; chemical, petrochemical and pharmaceutical industries; mineral, metal and pulp and paper industries; and a wide variety of manufacturing, utility, health, educational and government establishments.
Source: https://www.jobbank.gc.ca/marketreport/occupation/16318/ca

Transferable Skills
Now that you have explored atomic theory, consider the following question to further develop the Transferable Skill in Self-Directed Learning:
As we continue to learn more about atoms, ions, and isotopes, what significance may this have for modern medicine?
Self-Reflection
Read the following descriptions and select which one best describes your current level of comfort toward the learning concepts in this learning activity. Copy the descriptions and the description that you have selected into your notebook. When you review your notes on this learning activity later reflect on whether you would select a different description based on revising the learning material.
Rate your understanding on a scale of one to five.
Congratulations!
It’s time to celebrate! You have successfully completed the second learning activity in Unit 1. In the learning summary below, you will find the main points of this learning activity.

Learning summary
Here are some of the main points from this learning activity:
- The particle theory of matter can help us understand the composition of matter and the effect of energy on matter as it relates to solid, liquid, and gas states.
- There are three types of particles that make up matter: atoms, ions, and molecules.
- Atoms are the smallest unit of matter, and are made up of three types of subatomic particles: protons, neutrons, and electrons.
- The protons and neutrons make up the nucleus, while the electrons exist in the space around the nucleus.
- The atomic number is equal to the number of protons.
- In a neutral atom, the number of protons and electrons are equal.
- Protons and neutrons are relatively large compared to electrons, and together make up the mass number of a particular atom.
- The periodic table lists all elements in order of increasing atomic number.
- Atoms of the same element with different numbers of neutrons (and different masses) are called isotopes.
- The periodic table lists the average atomic mass for each element, which is determined based on the relative abundance of each of that element’s isotopes present in nature.
- Molecules are made up of two or more atoms bound together.
- Ions are atoms or molecules that are electrically charged.
Next steps
There is always more to learn! In the next learning activity of the course you will be going more in depth with the periodic table of elements in order to learn about the structures and properties of elements.